The Same Concepts Used to Describe the Emission and Absorption
The photons emitted in the stimulated emission process will travel in the same direction of the incident photon. In 1955 Alan Walsh from Australia applied the principle and instrumentation of atomic absorption spectroscopy for the.
Difference Between Emission And Absorption Spectra Comparison Chart
Absorption spectra also known as UV-Vis spectra absorbance spectra and electronic spectra show the change in absorbance of a sample as a function of the wavelength of incident light Figure 1 and are measured using a spectrophotometer.

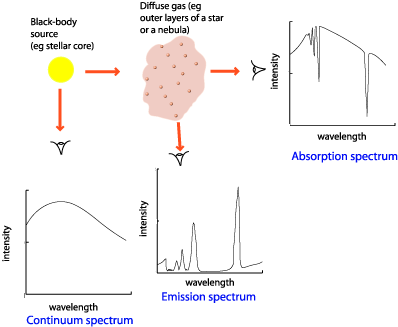
. Dark matt surfaces are poor reflectors and good at absorbing IR. The emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Atomic emission is the energy released from an excited atom as it goes back down to its ground state emitting discrete wavelengths.
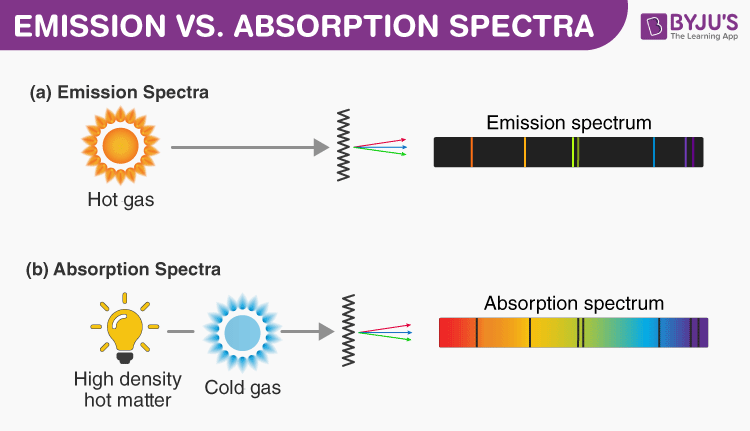
The gaps in the spectrum of the Sun get developed and help in informing the formation of the sun. Emission and Absorption Spectra. The absorption spectrum is the opposite of the emission spectrum.
In developing an absorption spectrum the light needs to shine through a gas but in creating and emission spectrum a gas needs to be heated up. Produced when atoms absorb energy. The chemical compound is heated in a flame and a colored flame is made.
It was first observed by Fraunhofer while studying dark lines in the solar spectrum. Emission refers to the release of energy by the electrons. It is the exact opposite of the emission spectrum.
Absorption spectra are measured by varying the wavelength of the incident light using a monochromator and recording the intensity. Atomic absorption spectroscopy AAS and atomic emission spectroscopy AES principle is based upon the absorption and emission of light by atoms in the gaseous state. Emission and Absorption Spectra.
During absorption an electron takes energy from an incoming photon and the internal energy of the electron increases. 408 explain how emission and absorption of radiation are related to surface and temperature. The same concepts used to describe the emission and absorption of virtual photons in electromagnetic interactions can be used to describe nuclear interactions.
Atoms molecules and ions that have absorbed radiation are called excited. Normally it is used to see whether there are certain metals in a chemical compound. The same concepts used to describe the emission and absorption of virtual photons in electromagnetic interactions can be used to describe nuclear interactions.
Stimulated emission occurs when a photon with energy equal to the energy gap of the levels interacts with the electron. Both emission and absorption techniques can be used to get the same information about the energy levels of an atom. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light.
Atomic Absorption and Emission Spectra. In stimulated emission process each incident photon generates two photons. The same concepts used to describe the emission and absorption of virtual photons in electromagnetic interaction can be used to describe nucleus interactions.
371 we includeinaccurate measurements of. This means that placed next to a heat source a dark object would heat up faster than a light one. Emission is when an electron is moving from a high energy state to a low energy state and energy is being released.
The radiation could be a function of either frequency or wavelength. A flame test is a test for chemicals. Atomic absorption is when an atom absorbs light to go from a ground state to an excited state.
An atom can absorb or emit one photon when an electron makes a transition from one stationary state or energy level to another. Absorption is when an electron is moved from a low energy state to a high energy state and energy is being absorbed. Absorption spectroscopy is a spectroscopic technique that is used for measuring the absorption of radiation as it interacts with the sample.
Can be used to figure out the ability of certain objects to retain heat and its absorption level. In order to effectively mediate the nuclear force the virtual particle must exist long. The emission spectrum is quite different from the absorption spectrum.
Show only certain frequencies. According to this theory when two protons of a nucleus interact with each other a virtual particle is created. Many ways exist to produce light but the stimulated emission is the only method known to produce coherent light beam of photons with the same frequency.
Comprise coloured lines in the spectrum. As we have noted in the section on the Bohr atom isolated atoms can absorb and emit packets of electromagnetic radiation having discrete energies dictated by. It is helpful in figuring out the composition of a certain matter.
Thesamewavelengthrange3200to3600Ainvacuumasincaesium 360 Bureau oj StandardsJournal of Research Vol8 vaporIn paperNo. Emission spectrum of Hydrogen. According to this theory when two protons of a nucleus interact with each other a virtual particle is created.
Absorption favours the increase of oxidation number through the process of ionization. According to this theory when two protons of a nucleus interact with each other a virtual particle is created. Towards Quantum Mechanical Model of Atom.
According to this theory when two protons of a nucleus interact with each other a virtual particle is created. The same concepts used to describe the emission and absorption of virtual photons in electromagnetic interactions can be used to describe nuclear interactions. The dark lines correspond to the frequencies of light that have been absorbed by the gas.
Comprise dark lines or gaps in the spectrum. The colored flame can be turned into its spectrum to. Absorption spectroscopy is related to the absorption spectrum because the sample used interacts with photons from the radiating field.
During spontaneous emission the internal energy of an electron decreases and a photon is emitted. When electrons emit energy they move down towards a lower energy level. Light shiny surfaces are good reflectors of IR and so are poor at absorbing it.
By exchanging a virtual photon two charged particles interact with each other and the observable effect of their interaction is either repulsion when the particles have the same charge or attraction when the particles have opposing charges Part B The same concepts used to describe the emission and absorption of virtual photons in electromagnetic interactions can be used to. Absorption spectrum of Hydrogen. Produced when atoms release energy.
Association with Oxidation numbers. When electrons absorb energy they move up towards a higher energy level.

Image Of Absorption Emission And Continuous Spectra Absorption Spectra Show Spectral Lines Continuous Spectra H Chemistry Jokes 8th Grade Science Emissions
No comments for "The Same Concepts Used to Describe the Emission and Absorption"
Post a Comment